| DEAR WIKIPEDIA READERS: To protect our independence, we'll never run ads. We take no government funds. We survive on donations averaging about $15. Now is the time we ask. If everyone reading this right now gave $3, our fundraiser would be done within an hour. We’re a small non-profit with costs of a top 5 website: servers, staff and programs. Wikipedia is something special. It is like a library or a public park where we can all go to think and learn. If Wikipedia is useful to you, take one minute to keep it online and ad-free another year. Thank you. |
Problems donating? | Other ways to give | Frequently asked questions | By donating, you are agreeing to our donor privacy policy. The Wikimedia Foundation is a nonprofit, tax-exempt organization. *Monthly payments will be debited by the Wikimedia Foundation until you notify us to stop. We'll send you an email receipt for each payment, which will include a link to easy cancellation instructions.
the fundraiser would be over in an hour. |
x
|
Non-equilibrium thermodynamics
From Wikipedia, the free encyclopedia
| Thermodynamics |
|---|

|
The thermodynamic study of non-equilibrium systems requires more general concepts than are dealt with by equilibrium thermodynamics. One fundamental difference between equilibrium thermodynamics and non-equilibrium thermodynamics lies in the behaviour of inhomogeneous systems, which require for their study knowledge of rates of reaction which are not considered in equilibrium thermodynamics of homogeneous systems. This is discussed below. Another fundamental difference is the difficulty in defining entropy in macroscopic terms for systems not in thermodynamic equilibrium.[2][3]
Contents
[hide]- 1 Overview
- 2 Basic concepts
- 3 Stationary states, fluctuations, and stability
- 4 Local thermodynamic equilibrium
- 5 Entropy in evolving systems
- 6 Flows and forces
- 7 The Onsager relations
- 8 Speculated thermodynamic extremum principles for energy dissipation and entropy production
- 9 Applications of non-equilibrium thermodynamics
- 10 See also
- 11 References
- 12 Further reading
- 13 External links
Overview[edit]
Non-equilibrium thermodynamics is a work in progress, not an established edifice. This article will try to sketch some approaches to it and some concepts important for it.Some concepts of particular importance for non-equilibrium thermodynamics include time rate of dissipation of energy (Rayleigh 1873,[4] Onsager 1931,[5] also[6][7]), time rate of entropy production (Onsager 1931),[5] thermodynamic fields,[8][9][10] dissipative structure,[11] and non-linear dynamical structure.[7]
Of interest is the thermodynamic study of non-equilibrium steady states, in which entropy production and some flows are non-zero, but there is no time variation.
One initial approach to non-equilibrium thermodynamics is sometimes called 'classical irreversible thermodynamics'.[3] There are other approaches to non-equilibrium thermodynamics, for example extended irreversible thermodynamics,[3][12] and generalized thermodynamics,[13] but they are hardly touched on in the present article.
Quasi-radiationless non-equilibrium thermodynamics of matter in laboratory conditions[edit]
According to Wildt[14] (see also Essex[15][16][17]), current versions of non-equilibrium thermodynamics ignore radiant heat; they can do so because they refer to laboratory quantities of matter under laboratory conditions with temperatures well below those of stars. At laboratory temperatures, in laboratory quantities of matter, thermal radiation is weak and can be practically nearly ignored. But, for example, atmospheric physics is concerned with large amounts of matter, occupying cubic kilometers, that, taken as a whole, are not within the range of laboratory quantities; then thermal radiation cannot be ignored.Local equilibrium thermodynamics[edit]
The terms 'classical irreversible thermodynamics'[3] and 'local equilibrium thermodynamics' are sometimes used to refer to a version of non-equilibrium thermodynamics that demands certain simplifying assumptions, as follows. The assumptions have the effect of making each very small volume element of the system effectively homogeneous, or well-mixed, or without an effective spatial structure, and without kinetic energy of bulk flow or of diffusive flux. Even within the thought-frame of classical irreversible thermodynamics, care[7] is needed in choosing the independent variables[18] for systems. In some writings, it is assumed that the intensive variables of equilibrium thermodynamics are sufficient as the independent variables for the task (such variables are considered to have no 'memory', and do not show hysteresis); in particular, local flow intensive variables are not admitted as independent variables; local flows are considered as dependent on quasi-static local intensive variables. (In other writings, local flow variables are considered; these might be considered as classical by analogy with the time-invariant long-term time-averages of flows produced by endlessly repeated cyclic processes; examples with flows are in the thermoelectric phenomena known as the Seebeck and the Peltier effects, considered by Kelvin in the nineteenth century and by Onsager in the twentieth.[19][20] These effects occur at metal junctions, which were originally effectively treated as two-dimensional surfaces, with no spatial volume, and no spatial variation.) Also it is assumed that the local entropy density is the same function of the other local intensive variables as in equilibrium; this is called the local thermodynamic equilibrium assumption[6][7][11][12][19][21][22][23] (see also Keizer (1987)[24]). Radiation is ignored because it is transfer of energy between regions, which can be remote from one another. In the classical irreversible thermodynamic approach, there is allowed very small spatial variation, from very small volume element to adjacent very small volume element, but it is assumed that the global entropy of the system can be found by simple spatial integration of the local entropy density; this means that spatial structure cannot contribute as it properly should to the global entropy assessment for the system. This approach assumes spatial and temporal continuity and even differentiability of locally defined intensive variables such as temperature and internal energy density. All of these are very stringent demands. Consequently, this approach can deal with only a very limited range of phenomena. This approach is nevertheless valuable because it can deal well with some macroscopically observable phenomena.Extended irreversible thermodynamics[edit]
Extended irreversible thermodynamics is a branch of non-equilibrium thermodynamics that goes outside the restriction to the local equilibrium hypothesis. The space of state variables is enlarged by including the fluxes of mass, momentum and energy and eventually higher order fluxes. The formalism is well-suited for describing high-frequency processes and small-length scales materials.Basic concepts[edit]
There are many examples of stationary non-equilibrium systems, some very simple, like a system confined between two thermostats at different temperatures or the ordinary Couette flow, a fluid enclosed between two flat walls moving in opposite directions and defining non-equilibrium conditions at the walls. Laser action is also a non-equilibrium process, but it depends on departure from local thermodynamic equilibrium and is thus beyond the scope of classical irreversible thermodynamics; here a strong temperature difference is maintained between two molecular degrees of freedom (with molecular laser, vibrational and rotational molecular motion), the requirement for two component 'temperatures' in the one small region of space, precluding local thermodynamic equilibrium, which demands that only one temperature be needed. Damping of acoustic perturbations or shock waves are non-stationary non-equilibrium processes. Driven complex fluids, turbulent systems and glasses are other examples of non-equilibrium systems.The mechanics of macroscopic systems depends on a number of extensive quantities. It should be stressed that all systems are permanently interacting with their surroundings, thereby causing unavoidable fluctuations of extensive quantities. Equilibrium conditions of thermodynamic systems are related to the maximum property of the entropy. If the only extensive quantity that is allowed to fluctuate is the internal energy, all the other ones being kept strictly constant, the temperature of the system is measurable and meaningful. The system's properties are then most conveniently described using the thermodynamic potential Helmholtz free energy (A = U - TS), a Legendre transformation of the energy. If, next to fluctuations of the energy, the macroscopic dimensions (volume) of the system are left fluctuating, we use the Gibbs free energy (G = U + PV - TS), where the system's properties are determined both by the temperature and by the pressure.
Non-equilibrium systems are much more complex and they may undergo fluctuations of more extensive quantities. The boundary conditions impose on them particular intensive variables, like temperature gradients or distorted collective motions (shear motions, vortices, etc.), often called thermodynamic forces. If free energies are very useful in equilibrium thermodynamics, it must be stressed that there is no general law defining stationary non-equilibrium properties of the energy as is the second law of thermodynamics for the entropy in equilibrium thermodynamics. That is why in such cases a more generalized Legendre transformation should be considered. This is the extended Massieu potential. By definition, the entropy (S) is a function of the collection of extensive quantities
 . Each extensive quantity has a conjugate intensive variable
. Each extensive quantity has a conjugate intensive variable 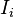 (a restricted definition of intensive variable is used here by comparison to the definition given in this link) so that:
(a restricted definition of intensive variable is used here by comparison to the definition given in this link) so that: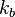 is Boltzmann's constant, whence
is Boltzmann's constant, whenceIntensities are global values, valid for the system as a whole. When boundaries impose to the system different local conditions, (e.g. temperature differences), there are intensive variables representing the average value and others representing gradients or higher moments. The latter are the thermodynamic forces driving fluxes of extensive properties through the system.
It may be shown that the Legendre transformation changes the maximum condition of the entropy (valid at equilibrium) in a minimum condition of the extended Massieu function for stationary states, no matter whether at equilibrium or not.
Stationary states, fluctuations, and stability[edit]
In thermodynamics one is often interested in a stationary state of a process, allowing that the stationary state include the occurrence of unpredictable and experimentally unreproducible fluctuations in the state of the system. The fluctuations are due to the system's internal sub-processes and to exchange of matter or energy with the system's surroundings that create the constraints that define the process.If the stationary state of the process is stable, then the unreproducible fluctuations involve local transient decreases of entropy. The reproducible response of the system is then to increase the entropy back to its maximum by irreversible processes: the fluctuation cannot be reproduced with a significant level of probability. Fluctuations about stable stationary states are extremely small except near critical points (Kondepudi and Prigogine 1998, page 323).[25] The stable stationary state has a local maximum of entropy and is locally the most reproducible state of the system. There are theorems about the irreversible dissipation of fluctuations. Here 'local' means local with respect to the abstract space of thermodynamic coordinates of state of the system.
If the stationary state is unstable, then any fluctuation will almost surely trigger the virtually explosive departure of the system from the unstable stationary state. This can be accompanied by increased export of entropy.
Local thermodynamic equilibrium[edit]
The scope of present-day non-equilibrium thermodynamics does not cover all physical processes. A condition for the validity of many studies in non-equilibrium thermodynamics of matter is that they deal with what is known as local thermodynamic equilibrium.Local thermodynamic equilibrium of ponderable matter[edit]
Local thermodynamic equilibrium of matter[6][11][21][22][23] (see also Keizer (1987)[24] means that conceptually, for study and analysis, the system can be spatially and temporally divided into 'cells' or 'micro-phases' of small (infinitesimal) size, in which classical thermodynamical equilibrium conditions for matter are fulfilled to good approximation. These conditions are unfulfilled, for example, in very rarefied gases, in which molecular collisions are infrequent; and in the boundary layers of a star, where radiation is passing energy to space; and for interacting fermions at very low temperature, where dissipative processes become ineffective. When these 'cells' are defined, one admits that matter and energy may pass freely between contiguous 'cells', slowly enough to leave the 'cells' in their respective individual local thermodynamic equilibria with respect to intensive variables.One can think here of two 'relaxation times' separated by order of magnitude.[26] The longer relaxation time is of the order of magnitude of times taken for the macroscopic dynamical structure of the system to change. The shorter is of the order of magnitude of times taken for a single 'cell' to reach local thermodynamic equilibrium. If these two relaxation times are not well separated, then the classical non-equilibrium thermodynamical concept of local thermodynamic equilibrium loses its meaning[26] and other approaches have to be proposed, see for instance Extended irreversible thermodynamics. For example, in the atmosphere, the speed of sound is much greater than the wind speed; this favours the idea of local thermodynamic equilibrium of matter for atmospheric heat transfer studies at altitudes below about 60 km where sound propagates, but not above 100 km, where, because of the paucity of intermolecular collisions, sound does not propagate.
Milne's 1928 definition of local thermodynamic equilibrium in terms of radiative equilibrium[edit]
Milne (1928),[27] thinking about stars, gave a definition of 'local thermodynamic equilibrium' in terms of the thermal radiation of the matter in each small local 'cell'. He defined 'local thermodynamic equilibrium' in a 'cell' by requiring that it macroscopically absorb and spontaneously emit radiation as if it were in radiative equilibrium in a cavity at the temperature of the matter of the 'cell'. Then it strictly obeys Kirchhoff's law of equality of radiative emissivity and absorptivity, with a black body source function. The key to local thermodynamic equilibrium here is that the rate of collisions of ponderable matter particles such as molecules should far exceed the rates of creation and annihilation of photons.Entropy in evolving systems[edit]
It is pointed out[28][29][30][31] by W.T. Grandy Jr that entropy, though it may be defined for a non-equilibrium system, is when strictly considered, only a macroscopic quantity that refers to the whole system, and is not a dynamical variable and in general does not act as a local potential that describes local physical forces. Under special circumstances, however, one can metaphorically think as if the thermal variables behaved like local physical forces. The approximation that constitutes classical irreversible thermodynamics is built on this metaphoric thinking.Flows and forces[edit]
The fundamental relation of classical equilibrium thermodynamics [32] of a system as a function of the intensive quantities temperature
of a system as a function of the intensive quantities temperature  , pressure
, pressure 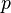 and
and  chemical potential
chemical potential  and of the differentials of the extensive quantities energy
and of the differentials of the extensive quantities energy  , volume
, volume 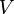 and
and  particle number
particle number 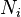 .
.Following Onsager (1931,I),[5] let us extend our considerations to thermodynamically non-equilibrium systems. As a basis, we need locally defined versions of the extensive macroscopic quantities
 ,
, 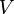 and
and 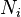 and of the intensive macroscopic quantities
and of the intensive macroscopic quantities  ,
, 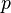 and
and  .
.For classical non-equilibrium studies, we will consider some new locally defined intensive macroscopic variables. We can, under suitable conditions, derive these new variables by locally defining the gradients and flux densities of the basic locally defined macroscopic quantities.
Such locally defined gradients of intensive macroscopic variables are called 'thermodynamic forces'. They 'drive' flux densities, perhaps misleadingly often called 'fluxes', which are dual to the forces. These quantities are defined in the article on Onsager reciprocal relations.
Establishing the relation between such forces and flux densities is a problem in statistical mechanics. Flux densities (
 ) may be coupled. The article on Onsager reciprocal relations considers the stable near-steady thermodynamically non-equilibrium regime, which has dynamics linear in the forces and flux densities.
) may be coupled. The article on Onsager reciprocal relations considers the stable near-steady thermodynamically non-equilibrium regime, which has dynamics linear in the forces and flux densities.In stationary conditions, such forces and associated flux densities are by definition time invariant, as also are the system's locally defined entropy and rate of entropy production. Notably, according to Ilya Prigogine and others, when an open system is in conditions that allow it to reach a stable stationary thermodynamically non-equilibrium state, it organizes itself so as to minimize total entropy production defined locally. This is considered further below.
One wants to take the analysis to the further stage of describing the behaviour of surface and volume integrals of non-stationary local quantities; these integrals are macroscopic fluxes and production rates. In general the dynamics of these integrals are not adequately described by linear equations, though in special cases they can be so described.
The Onsager relations[edit]
Main article: Onsager reciprocal relations
Following Section III of Rayleigh (1873),[4] Onsager (1931, I)[5] showed that in the regime where both the flows ( ) are small and the thermodynamic forces (
) are small and the thermodynamic forces (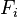 ) vary slowly, the rate of creation of entropy
) vary slowly, the rate of creation of entropy 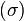 is linearly related to the flows:
is linearly related to the flows: :
: be positive definite. Statistical mechanics considerations involving microscopic reversibility of dynamics imply that the matrix
be positive definite. Statistical mechanics considerations involving microscopic reversibility of dynamics imply that the matrix  is symmetric. This fact is called the Onsager reciprocal relations.
is symmetric. This fact is called the Onsager reciprocal relations.Speculated thermodynamic extremum principles for energy dissipation and entropy production[edit]
Jou, Casas-Vazquez, Lebon (1993)[12] note that classical non-equilibrium thermodynamics "has seen an extraordinary expansion since the second world war", and they refer to the Nobel prizes for work in the field awarded to Lars Onsager and Ilya Prigogine. Martyushev and Seleznev (2006)[33] note the importance of entropy in the evolution of natural dynamical structures: "Great contribution has been done in this respect by two scientists, namely Clausius, ... , and Prigogine." Prigogine in his 1977 Nobel Lecture[34] said: "... non-equilibrium may be a source of order. Irreversible processes may lead to a new type of dynamic states of matter which I have called “dissipative structures”." Glansdorff and Prigogine (1971)[11] wrote on page xx: "Such 'symmetry breaking instabilities' are of special interest as they lead to a spontaneous 'self-organization' of the system both from the point of view of its space order and its function."Analyzing the Rayleigh-Bénard convection cell phenomenon, Chandrasekhar (1961)[35] wrote "Instability occurs at the minimum temperature gradient at which a balance can be maintained between the kinetic energy dissipated by viscosity and the internal energy released by the buoyancy force." With a temperature gradient greater than the minimum, viscosity can dissipate kinetic energy as fast as it is released by convection due to buoyancy, and a steady state with convection is stable. The steady state with convection is often a pattern of macroscopically visible hexagonal cells with convection up or down in the middle or at the 'walls' of each cell, depending on the temperature dependence of the quantities; in the atmosphere under various conditions it seems that either is possible. (Some details are discussed by Lebon, Jou, and Casas-Vásquez (2008)[3] on pages 143-158.) With a temperature gradient less than the minimum, viscosity and heat conduction are so effective that convection cannot keep going.
Glansdorff and Prigogine (1971)[11] on page xv wrote "Dissipative structures have a quite different [from equilibrium structures] status: they are formed and maintained through the effect of exchange of energy and matter in non-equilibrium conditions." They were referring to the dissipation function of Rayleigh (1873)[4] that was used also by Onsager (1931, I,[5] 1931, II[36]). On pages 78–80 of their book[11] Glansdorff and Prigogine (1971) consider the stability of laminar flow that was pioneered by Helmholtz; they concluded that at a stable steady state of sufficiently slow laminar flow, the dissipation function was minimum.
These advances have led to proposals for various extremal principles for the "self-organized" régimes that are possible for systems governed by classical linear and non-linear non-equilibrium thermodynamical laws, with stable stationary régimes being particularly investigated. Convection introduces effects of momentum which appear as non-linearity in the dynamical equations. In the more restricted case of no convective motion, Prigogine wrote of "dissipative structures". Šilhavý (1997)[37] offers the opinion that "... the extremum principles of [equilibrium] thermodynamics ... do not have any counterpart for [non-equilibrium] steady states (despite many claims in the literature)."
Prigogine’s proposed theorem of minimum entropy production[edit]
In 1945 Prigogine [38] (see also Prigogine (1947)[39]) proposed a “Theorem of Minimum Entropy Production” which applies only to the linear regime near a stationary thermodynamically non-equilibrium state. The proof offered by Prigogine is open to serious criticism.[7] A critical and unsupportive discussion of Prigogine's proposal is offered by Grandy (2008).[2] The rate of entropy production has been shown to be a non-monotonic function of time during the approach to steady state heat convection, which contradicts the proposal that it is an extremum in the optimum non-equilibrium state.[40]Speculated principles of maximum entropy production and minimum energy dissipation[edit]
Onsager (1931, I)[5] wrote: "Thus the vector field J of the heat flow is described by the condition that the rate of increase of entropy, less the dissipation function, be a maximum." Careful note needs to be taken of the opposite signs of the rate of entropy production and of the dissipation function, appearing in the left-hand side of Onsager's equation (5.13) on Onsager's page 423.[5]Although largely unnoticed at the time, Ziegler proposed an idea early with his work in the mechanics of plastics in 1961,[41] and later in his book on thermomechanics revised in 1983,[9] and in various papers (e.g., Ziegler (1987),[42]). Ziegler never stated his principle as a universal law but he may have intuited this. He demonstrated his principle using vector space geometry based on an “orthogonality condition” which only worked in systems where the velocities were defined as a single vector or tensor, and thus, as he wrote[9] at p. 347, was “impossible to test by means of macroscopic mechanical models”, and was, as he pointed out, invalid in “compound systems where several elementary processes take place simultaneously”.
In relation to the earth's atmospheric energy transport process, according to Tuck (2008),[43] "On the macroscopic level, the way has been pioneered by a meteorologist (Paltridge 1975,[44] 2001[45])." Initially Paltridge (1975)[44] used the terminology "minimum entropy exchange", but after that, for example in Paltridge (1978),[46] and in Paltridge (1979),[47] he used the now current terminology "maximum entropy production" to describe the same thing. The logic of Paltridge's earlier work is open to serious criticism.[2] Nicolis and Nicolis (1980) [48] discuss Paltridge's work, and they comment that the behaviour of the entropy production is far from simple and universal. Later work by Paltridge focuses more on the idea of a dissipation function than on the idea of rate of production of entropy.[45]
Sawada (1981),[49] also in relation to the earth's atmospheric energy transport process, postulating a principle of largest amount of entropy increment per unit time, cites work in fluid mechanics by Malkus and Veronis (1958)[50] as having "proven a principle of maximum heat current, which in turn is a maximum entropy production for a given boundary condition", but this inference is not logically valid. Again investigating planetary atmospheric dynamics, Shutts (1981)[51] used an approach to the definition of entropy production, different from Paltridge's, to investigate a more abstract way to check the principle of maximum entropy production, and reported a good fit.
Prospects[edit]
Until recently, prospects for useful extremal principles in this area have seemed clouded. C. Nicolis (1999)[52] concludes that one model of atmospheric dynamics has an attractor which is not a regime of maximum or minimum dissipation; she says this seems to rule out the existence of a global organizing principle, and comments that this is to some extent disappointing; she also points to the difficulty of finding a thermodynamically consistent form of entropy production. Another top expert offers an extensive discussion of the possibilities for principles of extrema of entropy production and of dissipation of energy: Chapter 12 of Grandy (2008)[2] is very cautious, and finds difficulty in defining the 'rate of internal entropy production' in many cases, and finds that sometimes for the prediction of the course of a process, an extremum of the quantity called the rate of dissipation of energy may be more useful than that of the rate of entropy production; this quantity appeared in Onsager's 1931[5] origination of this subject. Other writers have also felt that prospects for general global extremal principles are clouded. Such writers include Glansdorff and Prigogine (1971), Lebon, Jou and Casas-Vásquez (2008), and Šilhavý (1997), as noted in the Wikipedia article on Extremal principles in non-equilibrium thermodynamics.A recent proposal may perhaps by-pass those clouded prospects.[40]
Applications of non-equilibrium thermodynamics[edit]
Non-equilibrium thermodynamics has been successfully applied to describe biological systems such as Protein Folding/unfolding and transport through membranes.See also[edit]
- Dissipative system
- Entropy production
- Extremal principles in non-equilibrium thermodynamics
- Self-organization
- Autocatalytic reactions and order creation
- Self-organizing criticality
- Bogoliubov-Born-Green-Kirkwood-Yvon hierarchy of equations
- Boltzmann equation
- Vlasov equation
- Maxwell's daemon
- Information entropy
- Constructal theory
References[edit]
- Jump up ^ Fowler, R., Guggenheim, E.A. (1939). Statistical Thermodynamics, Cambridge University Press, Canbridge UK, page vii.
- ^ Jump up to: a b c d Grandy, W.T., Jr (2008). Entropy and the Time Evolution of Macroscopic Systems. Oxford University Press. ISBN 978-0-19-954617-6.
- ^ Jump up to: a b c d e Lebon, G., Jou, D., Casas-Vázquez, J. (2008). Understanding Non-equilibrium Thermodynamics: Foundations, Applications, Frontiers, Springer-Verlag, Berlin, e-ISBN 978-3-540-74252-4.
- ^ Jump up to: a b c Strutt, J. W. (1871). "Some General Theorems relating to Vibrations". Proceedings of the London Mathematical Society s1–4: 357–368. doi:10.1112/plms/s1-4.1.357.
- ^ Jump up to: a b c d e f g h Onsager, L. (1931). "Reciprocal relations in irreversible processes, I". Physical Review 37 (4): 405–426. Bibcode:1931PhRv...37..405O. doi:10.1103/PhysRev.37.405.
- ^ Jump up to: a b c Gyarmati, I. (1970). Non-equilibrium Thermodynamics. Field Theory and Variational Principles, translated by E. Gyarmati and W.F. Heinz, Springer, Berlin.
- ^ Jump up to: a b c d e Lavenda, B.H. (1978). Thermodynamics of Irreversible Processes, Macmillan, London, ISBN 0-333-21616-4.
- Jump up ^ Gyarmati, I. (1967/1970) Non-equilibrium Thermodynamics. Field Theory and Variational Principles, translated by E. Gyarmati and W.F. Heinz, Springer, New York, pages 4-14.
- ^ Jump up to: a b c Ziegler, H., (1983). An Introduction to Thermomechanics, North-Holland, Amsterdam, ISBN 0-444-86503-9.
- Jump up ^ Balescu, R. (1975). Equilibrium and Non-equilibrium Statistical Mechanics, Wiley-Interscience, New York, ISBN 0-471-04600-0, Section 3.2, pages 64-72.
- ^ Jump up to: a b c d e f Glansdorff, P., Prigogine, I. (1971). Thermodynamic Theory of Structure, Stability, and Fluctuations, Wiley-Interscience, London, 1971, ISBN 0-471-30280-5.
- ^ Jump up to: a b c Jou, D., Casas-Vázquez, J., Lebon, G. (1993). Extended Irreversible Thermodynamics, Springer, Berlin, ISBN 3-540-55874-8, ISBN 0-387-55874-8.
- Jump up ^ Eu, B.C. (2002). Generalized Thermodynamics. The Thermodynamics of Irreversible Processes and Generalized Hydrodynamics, Kluwer Academic Publishers, Dordrecht, ISBN 1-4020-0788-4.
- Jump up ^ Wildt, R. (1972). "Thermodynamics of the gray atmosphere. IV. Entropy transfer and production". Astrophysical Journal 174: 69–77. Bibcode:1972ApJ...174...69W. doi:10.1086/151469
- Jump up ^ Essex, C. (1984a). "Radiation and the irreversible thermodynamics of climate". Journal of the Atmospheric Sciences 41 (12): 1985–1991. Bibcode:1984JAtS...41.1985E. doi:10.1175/1520-0469(1984)041<1985:RATITO>2.0.CO;2.
- Jump up ^ Essex, C. (1984b). "Minimum entropy production in the steady state and radiative transfer". Astrophysical Journal 285: 279–293. Bibcode:1984ApJ...285..279E. doi:10.1086/162504
- Jump up ^ Essex, C. (1984c). "Radiation and the violation of bilinearity in the irreversible thermodynamics of irreversible processes". Planetary and Space Science 32 (8): 1035–1043. Bibcode:1984P&SS...32.1035E. doi:10.1016/0032-0633(84)90060-6
- Jump up ^ Prigogine, I., Defay, R. (1950/1954). Chemical Thermodynamics, Longmans, Green & Co, London, page 1.
- ^ Jump up to: a b De Groot, S.R., Mazur, P. (1962). Non-equilibrium Thermodynamics, North-Holland, Amsterdam.
- Jump up ^ Kondepudi, D. (2008). Introduction to Modern Thermodynamics, Wiley, Chichester UK, ISBN 978-0-470-01598-8, pages 333-338.
- ^ Jump up to: a b Balescu, R. (1975). Equilibrium and Non-equilibrium Statistical Mechanics, John Wiley & Sons, New York, ISBN 0-471-04600-0.
- ^ Jump up to: a b Mihalas, D., Weibel-Mihalas, B. (1984). Foundations of Radiation Hydrodynamics, Oxford University Press, New York, ISBN 0-19-503437-6.
- ^ Jump up to: a b Schloegl, F. (1989). Probability and Heat: Fundamentals of Thermostatistics, Freidr. Vieweg & Sohn, Brausnchweig, ISBN 3-528-06343-2.
- ^ Jump up to: a b Keizer, J. (1987). Statistical Thermodynamics of Nonequilibrium Processes, Springer-Verlag, New York, ISBN 0-387-96501-7.
- Jump up ^ Kondepudi, D., Prigogine, I, (1998). Modern Thermodynamics. From Heat Engines to Dissipative Structures, Wiley, Chichester, 1998, ISBN 0-471-97394-7.
- ^ Jump up to: a b Zubarev D. N.,(1974). Nonequilibrium Statistical Thermodynamics, translated from the Russian by P.J. Shepherd, New York, Consultants Bureau. ISBN 0-306-10895-X; ISBN 978-0-306-10895-2.
- Jump up ^ Milne, E.A. (1928). "The effect of collisions on monochromatic radiative equilibrium". Monthly Notices of the Royal Astronomical Society 88: 493–502. Bibcode:1928MNRAS..88..493M.
- Jump up ^ Grandy, W.T., Jr. (2004). "Time Evolution in Macroscopic Systems. I. Equations of Motion". Foundations of Physics 34: 1. arXiv:cond-mat/0303290. Bibcode:2004FoPh...34....1G. doi:10.1023/B:FOOP.0000012007.06843.ed.
- Jump up ^ Grandy, W.T., Jr. (2004). "Time Evolution in Macroscopic Systems. II. The Entropy". Foundations of Physics 34: 21. arXiv:cond-mat/0303291. Bibcode:2004FoPh...34...21G. doi:10.1023/B:FOOP.0000012008.36856.c1.
- Jump up ^ Grandy, W. T., Jr (2004). "Time Evolution in Macroscopic Systems. III: Selected Applications". Foundations of Physics 34 (5): 771. Bibcode:2004FoPh...34..771G. doi:10.1023/B:FOOP.0000022187.45866.81.
- Jump up ^ Grandy 2004 see also [1].
- Jump up ^ W. Greiner, L. Neise, and H. Stöcker (1997), Thermodynamics and Statistical Mechanics (Classical Theoretical Physics) ,Springer-Verlag, New York, P85, 91, 101,108,116, ISBN 0-387-94299-8.
- Jump up ^ Martyushev, L.M.; Seleznev, V.D. (2006). "Maximum entropy production principle in physics, chemistry and biology". Physics Reports 426: 1. Bibcode:2006PhR...426....1M. doi:10.1016/j.physrep.2005.12.001. Archived from the original on 2011-03-02.
- Jump up ^ Prigogine, I. (1977). Time, Structure and Fluctuations, Nobel Lecture.
- Jump up ^ Chandrasekhar, S. (1961). Hydrodynamic and Hydromagnetic Stability, Clarendon Press, Oxford.
- Jump up ^ Onsager, L. (1931). "Reciprocal relations in irreversible processes. II". Physical Review 38 (12): 2265–2279. Bibcode:1931PhRv...38.2265O. doi:10.1103/PhysRev.38.2265.
- Jump up ^ Šilhavý, M. (1997). The Mechanics and Thermodynamics of Continuous Media, Springer, Berlin, ISBN 3-540-58378-5, page 209.
- Jump up ^ Prigogine, I. (1945). "Modération et transformations irreversibles des systemes ouverts". Bulletin de la Classe des Sciences., Academie Royale de Belgique 31: 600–606.
- Jump up ^ Prigogine, I. (1947). Étude thermodynamique des Phenomènes Irreversibles, Desoer, Liege.
- ^ Jump up to: a b Attard, P. (2012). "Optimising Principle for Non-Equilibrium Phase Transitions and Pattern Formation with Results for Heat Convection". arXiv.
- Jump up ^ Ziegler, H. (1961). "Zwei Extremalprinzipien der irreversiblen Thermodynamik". Ingenieur-Archiv 30 (6): 410–416. doi:10.1007/BF00531783.
- Jump up ^ Ziegler, H.; Wehrli, C. (1987). "On a principle of maximal rate of entropy production". J. Non-Equilib. Thermodyn 12 (3): 229–243. Bibcode:1987JNET...12..229Z. doi:10.1515/jnet.1987.12.3.229.
- Jump up ^ Tuck, Adrian F. (2008) Atmospheric Turbulence: a molecular dynamics perspective, Oxford University Press. ISBN 978-0-19-923653-4. See page 33.
- ^ Jump up to: a b Paltridge, G. W. (1975). "Global dynamics and climate - a system of minimum entropy exchange". Quarterly Journal of the Royal Meteorological Society 101 (429): 475. Bibcode:1975QJRMS.101..475P. doi:10.1002/qj.49710142906.[dead link]
- ^ Jump up to: a b Paltridge, Garth W. (2001). "A physical basis for a maximum of thermodynamic dissipation of the climate system". Quarterly Journal of the Royal Meteorological Society 127 (572): 305. Bibcode:2001QJRMS.127..305P. doi:10.1002/qj.49712757203.
- Jump up ^ Paltridge, G. W. (1978). "The steady-state format of global climate". Quarterly Journal of the Royal Meteorological Society 104 (442): 927. Bibcode:1978QJRMS.104..927P. doi:10.1002/qj.49710444206.[dead link]
- Jump up ^ Paltridge, Garth W. (1979). "Climate and thermodynamic systems of maximum dissipation". Nature 279 (5714): 630. Bibcode:1979Natur.279..630P. doi:10.1038/279630a0.
- Jump up ^ Nicolis, G.; Nicolis, C. (1980). "On the entropy balance of the earth-atmosphere system". Quarterly Journal of the Royal Meteorological Society 125 (557): 1859–1878. Bibcode:1999QJRMS.125.1859N. doi:10.1002/qj.49712555718.
- Jump up ^ Sawada, Y. (1981). A thermodynamic variational principle in nonlinear non-equilibrium phenomena, Progress of Theoretical Physics 66: 68-76.
- Jump up ^ Malkus, W.V.R.; Veronis, G. (1958). "Finite amplitude cellular convection". Journal of Fluid Mechanics 4 (3): 225–260. Bibcode:1958JFM.....4..225M. doi:10.1017/S0022112058000410.
- Jump up ^ Shutts, G.J. (1981). "Maximum entropy production states in quasi-geostrophic dynamical models". Quarterly Journal of the Royal Meteorological Society 107 (453): 503–520. doi:10.1256/smsqj.45302.
- Jump up ^ Nicolis, C. (1999). "Entropy production and dynamical complexity in a low-order atmospheric model". Quarterly Journal of the Royal Meteorological Society 125 (557): 1859–1878. Bibcode:1999QJRMS.125.1859N. doi:10.1002/qj.49712555718.
Further reading[edit]
- Ziegler, Hans (1977): An introduction to Thermomechanics. North Holland, Amsterdam. ISBN 0-444-11080-1. Second edition (1983) ISBN 0-444-86503-9.
- Kleidon, A., Lorenz, R.D., editors (2005). Non-equilibrium Thermodynamics and the Production of Entropy, Springer, Berlin. ISBN 3-540-22495-5.
- Prigogine, I. (1955/1961/1967). Introduction to Thermodynamics of Irreversible Processes. 3rd edition, Wiley Interscience, New York.
- Zubarev D. N. (1974): Nonequilibrium Statistical Thermodynamics. New York, Consultants Bureau. ISBN 0-306-10895-X; ISBN 978-0-306-10895-2.
- Keizer, J. (1987). Statistical Thermodynamics of Nonequilibrium Processes, Springer-Verlag, New York, ISBN 0-387-96501-7.
- Zubarev D. N., Morozov V., Ropke G. (1996): Statistical Mechanics of Nonequilibrium Processes: Basic Concepts, Kinetic Theory. John Wiley & Sons. ISBN 3-05-501708-0.
- Zubarev D. N., Morozov V., Ropke G. (1997): Statistical Mechanics of Nonequilibrium Processes: Relaxation and Hydrodynamic Processes. John Wiley & Sons. ISBN 3-527-40084-2.
- Tuck, Adrian F. (2008). Atmospheric turbulence : a molecular dynamics perspective. Oxford University Press. ISBN 978-0-19-923653-4.
- Grandy, W.T., Jr (2008). Entropy and the Time Evolution of Macroscopic Systems. Oxford University Press. ISBN 978-0-19-954617-6.
- Kondepudi, D., Prigogine, I. (1998). Modern Thermodynamics: From Heat Engines to Dissipative Structures. John Wiley & Sons, Chichester. ISBN 0-471-97393-9.
- de Groot S.R., Mazur P. (1984). Non-Equilibrium Thermodynamics (Dover). ISBN 0-486-64741-2
External links[edit]
- Stephan Herminghaus' Dynamics of Complex Fluids Department at the Max Planck Institute for Dynamics and Self Organization
- Non-equilibrium Statistical Thermodynamics applied to Fluid Dynamics and Laser Physics - 1992- book by Xavier de Hemptinne.
- Nonequilibrium Thermodynamics of Small Systems - PhysicsToday.org
- Into the Cool - 2005 book by Dorion Sagan and Eric D. Schneider, on nonequilibrium thermodynamics and evolutionary theory.
- Quantum Thermodynamics - list of good related articles from the quantum thermodynamics point of view
- Thermodynamics ‘‘beyond’’ local equilibrium





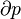

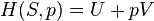

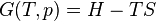
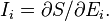
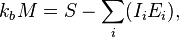
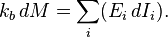

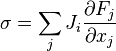
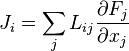
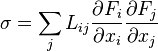
No comments:
Post a Comment