pdg.lbl.gov/.../reviews/rpp2011-...
2006” by P.J. Mohr, B.N. Taylor, and D.B. Newell in Rev. Mod. ... coupling constant) comes from the Particle Data Group. ... Planck constant, reduced ... wavelength of 1 eV/c particle hc/(1 eV). 1.239 841 875(31)×10−6 m. 25. Rydberg energy.
Lawrence Berkeley National Laboratory
Loading...
The Planck scale: relativity meets quantum mechanics ...
newt.phys.unsw.edu.au/einsteinlight/jw/module6_Planck.htm
The Planck length LP is defined by taking the constants of nature and ... that they can produce, and the latest generation produces energies of TeV, or 1012 eV.Photoelectric Effect - HyperPhysics
hyperphysics.phy-astr.gsu.edu/hbase/mod2.html
The minimum energy required to eject an electron from the surface is called the ... Using this wavelength in the Planck relationship gives a photon energy of 1.82 eV. ... The quantum of energy for a photon is not Planck's constant h itself, but the
Georgia State University
Loading...
Energy of Photon | PVEducation
www.pveducation.org › ... › 2. Properties of Sunlight
where h is Planck's constant and c is the speed of light. ... By expressing the equation for photon energy in terms of eV and µm we arrive at a commonly usedTime in Powers of Ten: Natural Phenomena and Their Timescales
https://books.google.com/books?isbn=9814494933
Gerard 't Hooft, Stefan Vandoren - 2014 - Science
PLANCK'S CONSTANT Position The constant of Planck is central to quantum ... For instance, for a particle with an energy of 7 TeV — at this moment the highest ...
Early Photoelectric Effect DataElectrons ejected from a sodium metal surface were measured as an electric current. Finding the opposing voltage it took to stop all the electrons gave a measure of the maximum kinetic energy of the electrons in electron volts.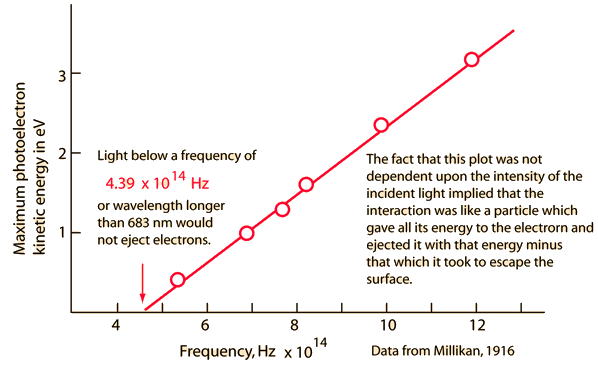 Further analysis
| Index Millikan reference Photoelectric effect | ||
| Go Back |
Early Photoelectric Effect Data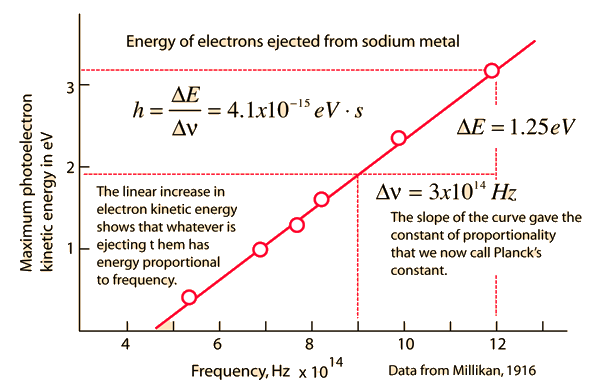 Planck hypothesis | Index Millikan reference Photoelectric effect | ||
| Go Back |
The Planck HypothesisIn order to explain the frequency distribution of radiation from a hot cavity (blackbody radiation) Planck proposed the ad hoc assumption that the radiant energy could exist only in discrete quanta which were proportional to the frequency. This would imply that higher modes would be less populated and avoid the ultraviolet catastrophe of the Rayleigh-Jeans Law.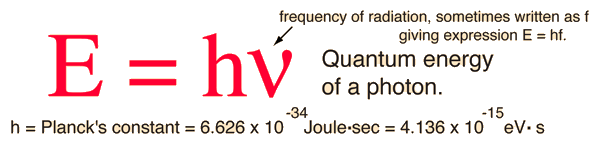 The quantum idea was soon seized to explain the photoelectric effect, became part of the Bohr theory of discrete atomic spectra, and quickly became part of the foundation of modern quantum theory.
| Index Photoelectric effect | |||||
| Go Back |
Photon Energies for EM Spectrum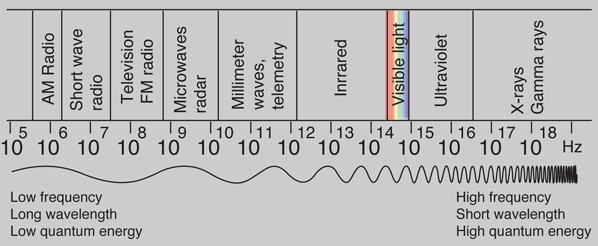
| Index | ||||
| Go Back |
Photons: The Quanta of LightAccording to the Planck hypothesis, all electromagnetic radiation is quantized and occurs in finite "bundles" of energy which we call photons. The quantum of energy for a photon is not Planck's constant h itself, but the product of h and the frequency. The quantization implies that a photon of blue light of given frequency or wavelength will always have the same size quantum of energy. For example, a photon of blue light of wavelength 450 nm will always have 2.76 eV of energy. It occurs in quantized chunks of 2.76 eV, and you can't have half a photon of blue light - it always occurs in precisely the same sized energy chunks.But the frequency available is continuous and has no upper or lower bound, so there is no finite lower limit or upper limit on the possible energy of a photon. On the upper side, there are practical limits because you have limited mechanisms for creating really high energy photons. Low energy photons abound, but when you get below radio frequencies, the photon energies are so tiny compared to room temperature thermal energy that you really never see them as distinct quantized entities - they are swamped in the background. Another way to say it is that in the low frequency limits, things just blend in with the classical treatment of things and a quantum treatment is not necessary. 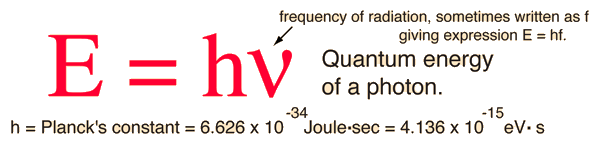
| Index Photoelectric effect | ||||
| Go Back |
No comments:
Post a Comment